INCREASED health awareness and literacy, especially after the Covid-19 pandemic, has led to Digital Therapeutics (DTx) becoming an innovative area in the life science and healthcare industry.
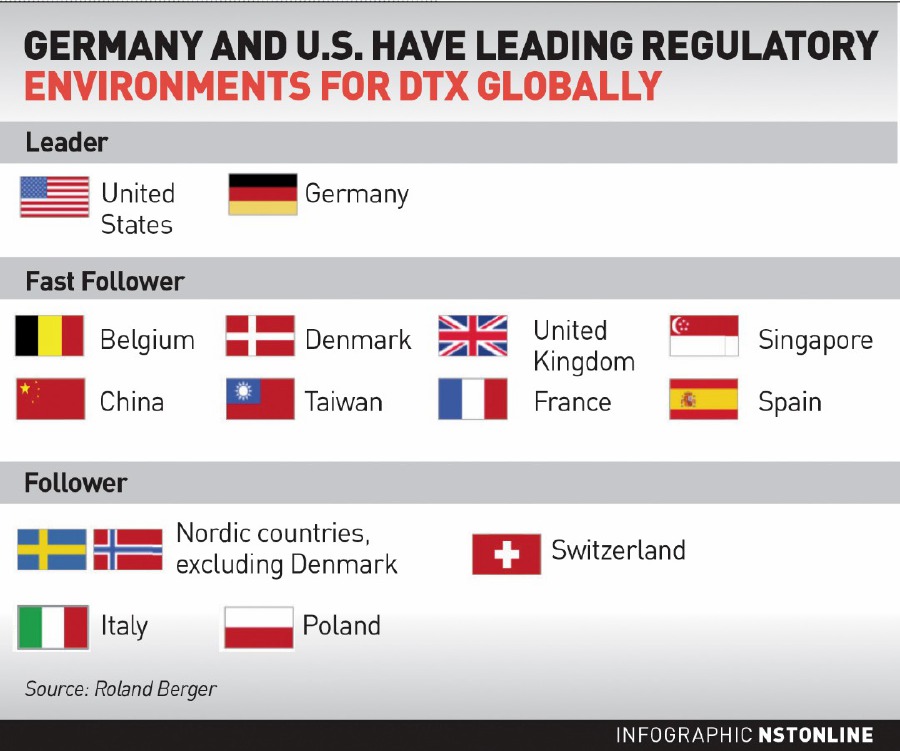
In a recent study conducted by management consultancy Roland Berger, the company found that the United States and Germany were seen as global leaders in providing strong support and incentives to develop DTx, while Singapore, China, and Taiwan were seen as 'Fast Followers' among Asian countries.
As the sector is in its early stage, DTx startups and investors are looking to find the right countries to develop and launch their programmes.
Malaysia also has the potential to be a hub in Asia by providing a clear policy framework and inviting DTx startups to the country.
"There is no clear DTx hub country in Asia, and Malaysia has the potential to be there by preparing the regulatory infrastructure and startup ecosystem.
"This would eventually benefit local pharmaceutical companies as well," said Roland Berger partner Life Sciences and Healthcare Southeast Asia Yoshihiro Suwa, who is also the author of the study.

DTx is expected to be a game changer in the treatment of diseases.
There is long list of approved DTx examples across the patient journey, mainly at screening / diagnosis, treatment (as monotherapy), and treatment support (as combination therapy).
For example, nervous system diseases, such as ADHD, schizophrenia, and depression, are the diseases which DTx is seen as a potential replacement of drug treatment.

DTx also functions to help improve drug adherence by recording and monitoring the conditions through the smartphone app.
It is also developed for key diseases such as oncology.
For Malaysia to be a DTx hub and attract investors and startups across the world, it first requires a policy framework to cover the following aspects:
• Regulatory approval: to set the DTx definition, evaluation process / procedures, and requirement for the approval
• Reimbursement: to set the process and requirements for universal healthcare coverage
• Startup ecosystem: to develop favourable incentives and ecosystem to support startups to have access to investors, develop DTx, and conduct clinical studies with support from medical providers.
In this area, Germany is one of the global leaders which has a well-structured policy framework for DTx, which Malaysia can learn from.
The country introduced a Digital Health Act (DVG) in 2019 to define an application process, so called 'fast track', where solution providers can apply for being certificated as DTx.
They also developed an official reimbursement directory where certified DTx are listed, with a clear criteria that solutions have to fulfill to achieve the certification (e.g. proof of positive health effect).
The country also prepared the framework for agreement between the German Federal Association of Health Insurance Funds (GKV-SV) and the manufacturers of DTx on reimbursement amounts.
With the introduction of the DVG, 73 million publicly insured people in Germany were able to access to DTx.
At the same time, prescribers can access the evaluated apps at an official reimbursement directory.
Thanks to the readiness of the policy framework, Germany has attract DTx startups and investors. Up to December last year, more than 30 apps were listed in DTx directory as reimbursable by the publicly insured population.
Investment in DTx may also help local pharmaceutical companies tranform their business.
Based on the Berger survey, global pharmaceutical companies see five reasons to engage in DTx:
• DTx as standalone business: it is expected to be a sizeable and strongly growing market across the world with high recurring revenues in the future.
• Adherence push: DTx will help to push the adherence rates of patients by intelligent reminder functions. This would increase the sales of drug portfolios.
• Co-marketing effect: Selling DTx via own sales force could open up new accounts for existing therapies, as innovative treatment attracts innovative prescribers.
• Real-world evidence (RWE): Create supporting data/evidence for current drugs by use of DTx in broad population and real world setting.
• Lighthouse effect: global / regional recognition as an innovation leader in DTx with potential to scale to other markets.
French pharmaceutical company Servier is one of the examples that benefited from being an innovator of DTx.

The company acquired a licence for the German market to offer Deprexis, a DTx platform to support the treatment of cancer, as early as 2015.
In 2016, Servier launched an e-health division called WeHealth to accelerate digital initiatives.
Since then, the company has conducted trials to develop real world evidence to validate treatment
effectiveness as well as health economics.
Due to these efforts, Servier is now able to attract lots of startups for collaboration in DTx. The launch of Cardioskin together with BioSerenity in 2019 were two other collaborations initiated by Servier.